Thymosin Alpha-1 Research
Summarization
Thymosin Alpha-1 (Tα1) is a naturally occurring substance in the body that is being researched for its role in regulating the immune system, particularly in boosting its function when it’s weakened. It works by activating key immune cells, like dendritic cells, which then help other important immune cells, called T-cells, to effectively combat infections and even cancer.
Extensive research has shown that Tα1 is highly effective as a “vaccine helper”, meaning it can significantly improve how well vaccines work, especially in older individuals or those with compromised immune systems. It helps their bodies produce more protective antibodies against diseases.
However, when it comes to treating more serious conditions such as various cancers or severe infections like sepsis (a life-threatening response to infection) and COVID-19, the results have been mixed. While some studies have shown promise, larger, more comprehensive trials haven’t consistently demonstrated a clear benefit across all patients. This suggests that Tα1 might be most beneficial for specific groups of patients whose immune systems are struggling in particular ways.
Despite its use in over 35 countries, Tα1 is not approved by the U.S. Food and Drug Administration (FDA). This is largely due to concerns about the consistency of its effectiveness in large-scale studies and issues related to the quality control of versions produced by certain compounding pharmacies.
Nevertheless, Tα1 has an excellent safety record, with very few side effects observed over decades of clinical use. Future research is expected to focus on identifying specific immune indicators in patients that can help predict who would most likely benefit from Tα1 treatment, leading to a more targeted and effective approach.
Abstract
Thymosin Alpha-1 (Tα1) is a 28-amino acid peptide, originally isolated from the thymus gland, that has garnered significant scientific interest for its pleiotropic immunomodulatory properties. As a biological response modifier, Tα1 plays a pivotal role in restoring and enhancing immune function, particularly in states of immunosuppression. The primary mechanism of action involves interaction with Toll-like receptors (TLRs) on innate immune cells, such as dendritic cells, which initiates a MyD88-dependent signaling cascade. This cascade activates key downstream pathways, including p38 MAPK and NF-κB, leading to dendritic cell maturation and the orchestration of a T-helper 1 (Th1) polarized adaptive immune response, which is critical for antiviral and antitumor immunity.
This review synthesizes the extensive body of preclinical and clinical research on Tα1. Clinical evidence strongly supports its efficacy as a vaccine adjuvant, particularly in elderly and immunocompromised populations, where it significantly enhances antibody titers and reduces infection rates. Its application in oncology and infectious diseases presents a more complex picture, with studies showing promising but often inconsistent results, highlighting the critical influence of the host’s underlying immune status.
Large, randomized controlled trials in heterogeneous populations, such as sepsis, have failed to demonstrate a clear mortality benefit, yet subgroup analyses suggest potential efficacy in specific patient cohorts. Conversely, its safety profile is exceptionally favorable, with decades of clinical use demonstrating excellent tolerability and a low incidence of adverse events. Despite its approval in over 35 countries, Tα1 remains unapproved by the U.S. Food and Drug Administration (FDA), a regulatory paradox stemming from concerns over inconsistent efficacy data in large trials and the quality of compounded preparations.
This comprehensive analysis concludes that the future of Tα1 therapy likely resides in a stratified medicine approach, utilizing immune biomarkers to identify patient populations with defined immune deficits who are most poised to benefit from its restorative immunomodulatory effects.
1. Introduction: From Thymic Extract to Immunomodulatory Peptide
1.1 The Discovery and Isolation of Thymosin Alpha-1
The discovery of Thymosin Alpha-1 (Tα1) is rooted in foundational immunology research from the 1960s. Seminal experiments demonstrated that neonatal thymectomy, the surgical removal of the thymus gland in newborn animals, had profound and devastating consequences on the developing immune system. These animals exhibited a marked deficiency in lymphocyte populations (a critical type of white blood cell), an inability to mount cell-mediated immune responses such as graft rejection, and a failure to produce antibodies against certain antigens. This state of severe immunodeficiency often culminated in a fatal condition known as “wasting disease”. A pivotal observation was that these effects could be reversed by transplanting thymic tissue, even when the tissue was enclosed in a cell-impermeable chamber. This suggested that the thymus produced one or more soluble, hormone-like factors responsible for orchestrating immune system development and function.
This hypothesis spurred a concerted effort to isolate and characterize these thymic factors. In the mid-1960s, Goldstein et al. first reported the partial purification of a thymic extract, which they named “thymosin.” This extract was shown to induce lymphocytopoiesis (the formation of lymphocytes), prevent wasting disease, and restore immunological competence in thymectomized mice. Initial preparations, known as thymosin fraction 3, were further refined to yield a more potent and purified preparation called thymosin fraction. This fraction, a complex mixture of at least 40 distinct polypeptides, was stable, could be produced in larger quantities, and was deemed suitable for initial clinical investigations.
1.2 Biochemical Characterization
The individual polypeptides within thymosin fraction 5 were categorized based on their isoelectric point (pI), which is the specific pH at which a molecule carries no net electrical charge. This classification scheme gave rise to three main groups: the α-fractions (pI < 5.0), β-fractions (pI 5.0–7.0), and γ-fractions (pI > 7.0). In 1979, the first individual peptide was successfully isolated and sequenced from the highly acidic α-fraction. This peptide was named Thymosin Alpha-1 (Tα1).
Biochemically, Tα1 is an N-terminally acetylated polypeptide composed of 28 amino acids, with a molecular weight of 3,108 Daltons. The N-terminal acetylation is a post-translational modification that serves as a protective cap, enhancing the peptide’s stability against degradation by cellular enzymes. The synthetic analog of Tα1, known as thymalfasin, is chemically identical to the naturally occurring human peptide and is the form used in the vast majority of modern clinical trials and therapeutic applications. Thymalfasin is approved for clinical use in more than 35 countries worldwide for various indications.
1.3 Overview of Pleiotropic Immunomodulatory Functions
Tα1 is recognized not as a simple immune stimulant but as a pleiotropic immunomodulator, meaning it exerts multiple effects on various components of the immune system to restore homeostasis (a state of stable equilibrium).9 Its primary role is to modify, enhance, and restore immune function, particularly in states of immunodeficiency or dysregulation.
The peptide’s broad range of activities includes enhancing the maturation and function of T-cells and dendritic cells (DCs), modulating the production of cytokines and chemokines (the signaling molecules that orchestrate immune responses), and blocking steroid-induced apoptosis (a form of programmed cell death) of thymocytes, which are T-cells developing within the thymus. This multifaceted mechanism of action forms the basis for its investigation and application across a wide spectrum of pathological conditions, from infectious diseases and cancer to its use as a vaccine adjuvant, which will be explored in detail in the subsequent sections of this review.
2. Molecular Mechanism of Action: Orchestrating the Immune Response
The diverse biological effects of Tα1 are not the result of disparate, independent actions but rather a coordinated sequence of events that bridges the innate and adaptive branches of the immune system. The primary interaction of Tα1 occurs with the sentinels of the innate immune system, particularly dendritic cells. This initial activation step sets in motion a cascade of signaling events that ultimately shapes and amplifies the highly specific, long-lasting response of the adaptive immune system, most notably T-lymphocytes. This hierarchical mechanism explains how a single peptide can orchestrate a complex, multifaceted immune response tailored to combat both pathogens and malignancies.
2.1 Interaction with the Innate Immune System
2.1.1 Targeting Toll-Like Receptors (TLRs) on Dendritic Cells

The innate immune system provides the body’s first line of defense against pathogens. A key component of this system is a family of proteins known as Toll-like receptors (TLRs). TLRs are pattern recognition receptors (PRRs) located on the surface or within intracellular compartments (endosomes) of innate immune cells like dendritic cells and macrophages. Their primary function is to recognize conserved molecular structures, known as pathogen-associated molecular patterns (PAMPs), which are unique to microorganisms (such as lipopolysaccharide from bacterial cell walls or double-stranded RNA from viruses), as well as damage-associated molecular patterns (DAMPs) released from damaged host cells. This recognition event is the critical first step in initiating an inflammatory and defensive response.
Tα1 functions as a novel signaling molecule that directly engages this system. It acts as an agonist (a substance that initiates a physiological response when combined with a receptor) for specific TLRs expressed on both myeloid and plasmacytoid dendritic cells, which are the most potent antigen-presenting cells in the body. Research has specifically demonstrated that Tα1 can directly activate TLR9, an endosomal receptor that typically recognizes unmethylated CpG DNA motifs found in bacteria and viruses. Furthermore, Tα1 has been shown to potentiate, or enhance, the signaling through TLR2, a surface receptor that recognizes peptidoglycan and other components of bacterial cell walls. By interacting with multiple TLRs, Tα1 primes the innate immune system to mount a more robust and comprehensive response to a wide array of microbial threats.
2.1.2 Activation of Downstream Signaling Cascades
The binding of Tα1 to its cognate TLRs triggers a well-defined intracellular signaling cascade. This process begins with the recruitment of a crucial adaptor protein known as Myeloid Differentiation primary response 88 (MyD88) to the cytoplasmic tail of the TLR. The engagement of MyD88 is the central event that initiates the MyD88-dependent signaling pathway, a cornerstone of innate immune activation.
Activation of the MyD88 pathway unleashes a series of phosphorylation events (the enzyme-mediated addition of phosphate groups to proteins, which functions as a molecular “on” switch). This cascade culminates in the activation of two critical downstream signaling hubs: p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor-kappa B (NF-κB).
The p38 MAPK pathway is a key signaling route involved in cellular responses to stress and inflammation, while NF-κB is a master transcription factor (a protein that controls the rate of transcription of genetic information from DNA to messenger RNA) that translocates into the cell’s nucleus. Once in the nucleus, NF-κB binds to specific DNA sequences to switch on the expression of a wide array of genes essential for inflammation, immune cell activation, and cell survival.
The functional consequence of activating these pathways in dendritic cells is a process known as maturation. Tα1-stimulated DCs upregulate the expression of molecules essential for initiating the adaptive immune response. These include Major Histocompatibility Complex (MHC) class I and class II molecules, which are responsible for presenting processed antigens (small fragments of pathogens or tumor proteins) to T-cells, and co-stimulatory molecules such as CD40, CD80, and CD86. These co-stimulatory molecules provide a critical “second signal” that is required, in addition to antigen presentation, to fully activate a naive T-cell and prevent it from becoming anergic (unresponsive).
2.2 Modulation of the Adaptive Immune Response
2.2.1 Promoting T-Cell Differentiation and Maturation
The activation and maturation of dendritic cells by Tα1 provides the crucial link to the adaptive immune system. These mature DCs migrate from peripheral tissues to lymph nodes, where they become highly efficient at presenting antigens to and activating naive T-lymphocytes. The effects of Tα1 on the adaptive immune system are therefore both direct and indirect, culminating in the enhanced differentiation and maturation of T-cells into their fully functional effector forms.3
This process results in an increased number and enhanced function of both CD4+ T-helper cells, which act as the “conductors” of the immune orchestra by coordinating the activities of other immune cells, and CD8+ cytotoxic T-lymphocytes (CTLs), often referred to as “killer” T-cells, which are responsible for directly identifying and eliminating virally infected cells and malignant tumor cells.3 The T-cell response is further amplified by Tα1’s ability to increase the expression of receptors for Interleukin-2 (IL-2) on the surface of T-cells. IL-2 is the primary cytokine responsible for promoting T-cell proliferation and survival, and by making T-cells more sensitive to its effects, Tα1 ensures a more robust and sustained adaptive immune response.7
2.2.2 Shaping the Cytokine Milieu: The T-helper 1 (Th1) Response
Thymosin Alpha-1 research as shown the type of adaptive immune response generated is heavily influenced by the cytokine environment created by activated innate cells. Tα1-matured dendritic cells predominantly secrete Interleukin-12 (IL-12), a powerful cytokine that is the primary driver for the differentiation of naive CD4+ T-cells into the T-helper 1 (Th1) subtype.15
A Th1-polarized immune response is specialized for combating intracellular pathogens, such as viruses and certain bacteria, as well as for tumor immunosurveillance. This response is characterized by the production of a specific set of cytokines, most notably Interferon-gamma (IFN-γ) and IL-2. IFN-γ is a potent activator of macrophages and enhances the cell-killing capacity of CD8+ T-cells and Natural Killer (NK) cells, while IL-2, as mentioned, drives T-cell proliferation.3 By promoting a Th1 phenotype, Tα1 tailors the adaptive immune response toward a cell-mediated pathway that is most effective for clearing viral infections and eliminating cancerous cells.
2.2.3 Enhancement of Natural Killer (NK) Cell Cytotoxicity
In addition to its effects on dendritic cells and T-cells, Thymosin Alpha-1 research has also shown it directly impacts the function of Natural Killer (NK) cells. NK cells are a component of the innate immune system, classified as innate lymphoid cells, that provide a rapid, non-specific defense against cellular threats. They are capable of recognizing and killing stressed, infected, or transformed (cancerous) cells without prior sensitization. Clinical and preclinical studies have consistently shown that Tα1 enhances the cytotoxic (cell-killing) activity of NK cells, providing another vital mechanism that contributes to its overall antiviral and antitumor efficacy.4
3. Clinical Research and Applications
The clinical investigation of Tα1 spans several decades and covers a wide range of conditions, from cancer and infectious diseases to its use as a vaccine adjuvant. The extensive body of evidence reveals a notable dichotomy: while Tα1 demonstrates a consistent and robust signal of efficacy in certain well-defined contexts, such as enhancing vaccine responses in immunocompromised individuals, its performance in large, heterogeneous patient populations has often been disappointing. This pattern of results suggests that the therapeutic efficacy of Tα1 is not universal but is critically dependent on the specific clinical indication and, more importantly, the underlying immune status of the patient. The failure of broad-based clinical trials does not necessarily indicate a lack of bioactivity but rather points toward the need for a more refined, stratified approach to its clinical application. Identifying and selecting patient populations with specific immune deficits, such as lymphocytopenia or immunosenescence, may be the key to unlocking the full therapeutic potential of this immunomodulatory peptide.
3.1 Oncology: Tα1 as an Adjuvant Therapy
The rationale for using Tα1 in oncology is twofold: to directly enhance the host’s antitumor immune response and to mitigate the immunosuppressive side effects of conventional treatments like chemotherapy and radiotherapy.2
3.1.1 Metastatic Melanoma
One of the most significant clinical investigations of Thymosin Alpha-1 research in oncology was a large, randomized, multicenter study involving 488 patients with metastatic melanoma.23 This trial evaluated Tα1 in combination with the chemotherapeutic agent dacarbazine (DTIC) and interferon-alfa (IFN-α). While the study did not meet its primary endpoint of best overall response in the broad intent-to-treat population, a more nuanced analysis of the data revealed important signals of activity. Specifically, the treatment arms containing Tα1 at a dose of 3.2 mg showed numerically higher tumor response rates compared to the control group (12 responses in the DTIC+Tα1 arm versus only 4 in the control arm).23
Furthermore, there was a clinically meaningful trend toward improved overall survival (OS) in patients receiving any Tα1-containing regimen compared to the control group (median OS of 9.4 months vs. 6.6 months, respectively).23 These findings, while not statistically definitive in the overall trial, suggested that Tα1 possesses biological activity against metastatic melanoma and provided a strong rationale for its further evaluation. This clinical potential is supported by preclinical models, where Tα1 monotherapy was shown to significantly decrease the formation of lung metastases. Importantly, these models also demonstrated that low, otherwise ineffective doses of Tα1 could synergize with anti-PD-1 checkpoint inhibitors to enhance their anti-metastatic efficacy, highlighting a potential role for Tα1 in modern combination immunotherapy regimens.2
3.1.2 Non-Small Cell Lung Cancer (NSCLC)
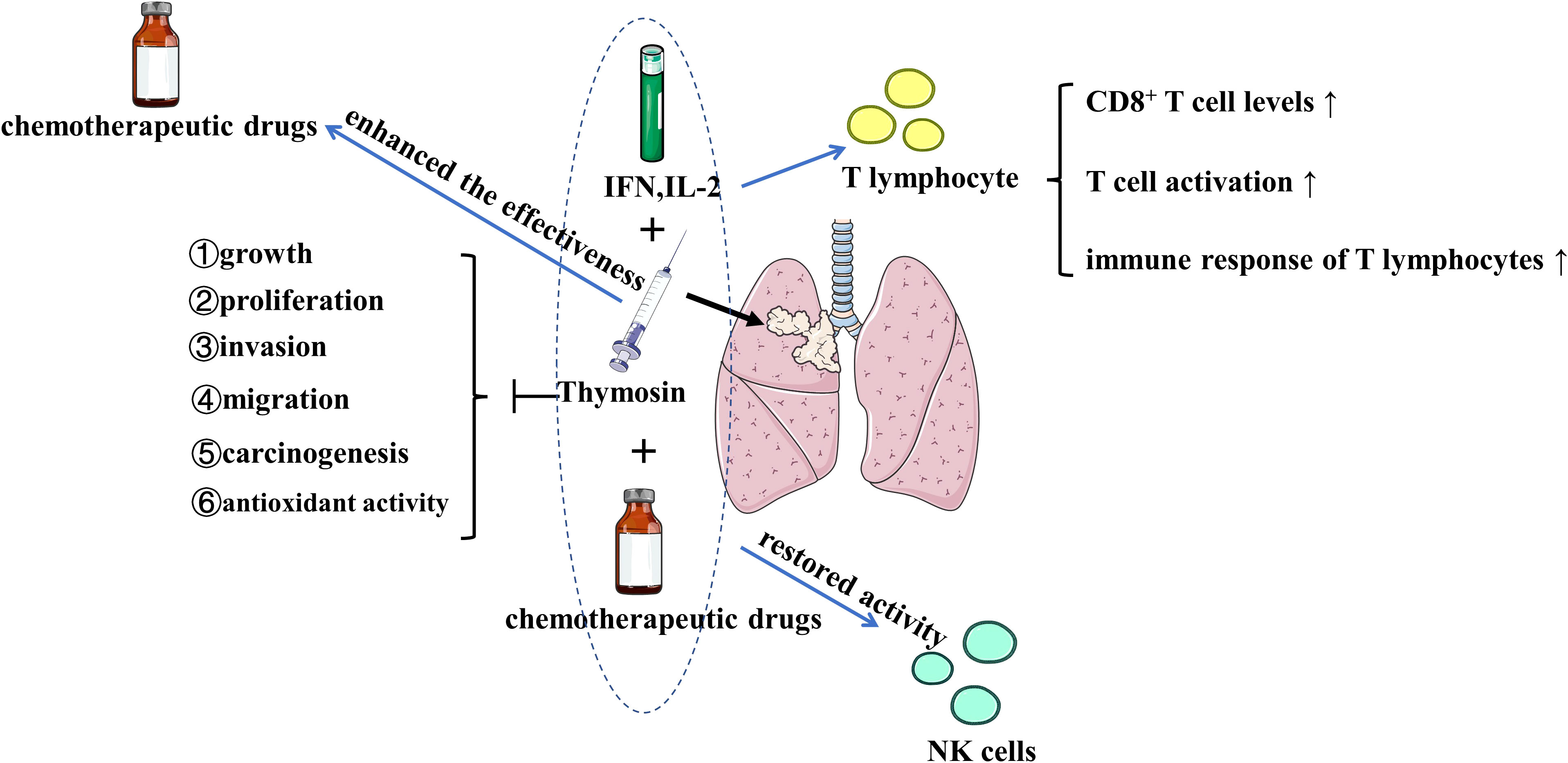
In non-small cell lung cancer (NSCLC), Tα1 has been shown to exert its effects through a dual mechanism of action.
First, it has direct anti-tumor properties. Tα1 can inhibit the phosphorylation of STAT3 (Signal Transducer and Activator of Transcription 3), a key signaling protein often overactive in cancer. This inhibition leads to decreased expression of matrix metalloproteinase 2 (MMP2), an enzyme that cancer cells use to degrade the extracellular matrix and facilitate invasion and migration.4
Second, Thymosin Alpha-1 research has shown it exerts powerful immunomodulatory effects within the tumor microenvironment. It has been shown to restore the cytotoxic activity of NK cells following chemotherapy and to increase the infiltration of CD4+ and CD8+ T-cells into tumor tissue.4
However, the immunomodulatory effects of Tα1 are complex. Thymosin Alpha-1 research has revealed that through its interaction with TLRs on dendritic cells, it can also upregulate Arginase 1, an enzyme that activates myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of immature myeloid cells that potently suppress T-cell responses and are a major mechanism of tumor immune evasion.4 This finding provides a compelling molecular explanation for why the clinical efficacy of Tα1 is often most pronounced when it is used in combination with cytotoxic chemotherapy or other immunotherapies. By combining Tα1 with agents that can deplete or overcome the suppressive effects of MDSCs, a more potent and synergistic antitumor immune response can be achieved.
3.2 Infectious Diseases: A Complex Picture of Efficacy

Thymosin Alpha-1: An Immunomodulating Peptide
3.2.1 Sepsis and Critical Illness
The investigation of Tα1 in sepsis provides a stark example of the challenges in translating promising early data into definitive clinical benefit. Sepsis is characterized by a dysregulated immune response to infection, often involving an initial hyper-inflammatory phase followed by a profound state of immunosuppression, which increases the risk of secondary infections and mortality. Given Tα1’s ability to restore immune function, it was considered a promising candidate for treating sepsis-induced immunosuppression.
Initial evidence was encouraging. A meta-analysis of 10 small randomized controlled trials, encompassing a total of 530 patients, suggested that Tα1 treatment was associated with a remarkable 41% relative reduction in 28-day mortality compared to control groups (22% vs. 38%).25 However, the quality of this evidence was rated as low due to the small sample sizes and methodological limitations of the included trials.25
To provide a more definitive answer, the large, multicenter, double-blind, randomized, placebo-controlled TESTS trial was conducted, enrolling 1089 adults with sepsis.25 The results of this robust trial were unequivocal: there was no statistically significant difference in the primary outcome of 28-day all-cause mortality between the Tα1 group (23.4%) and the placebo group (24.1%).25 This finding demonstrated that, in a broad, unselected population of patients with sepsis, Tα1 does not confer a survival benefit.
However, a prespecified subgroup analysis from the TESTS trial yielded intriguing, hypothesis-generating results. The analysis suggested a potential interaction between treatment effect and both age and the presence of diabetes. Tα1 appeared to be associated with potential harm in patients younger than 60, but showed a trend toward benefit in those aged 60 and older. More strikingly, Tα1 was associated with a statistically significant reduction in mortality among patients with diabetes (hazard ratio 0.58), while showing no benefit in patients without diabetes.25 These findings strongly support the concept that the efficacy of Tα1 is not uniform across all patients and that future studies should focus on specific, stratified populations, such as older adults or those with chronic comorbidities, who may have pre-existing immune deficits and are therefore more likely to benefit from an immunomodulatory therapy.
3.2.2 Chronic Viral Infections (Hepatitis B/C, HIV)
Historically, thymalfasin (the synthetic form of Tα1) was approved and used in many countries for the treatment of chronic hepatitis B (HBV) and hepatitis C (HCV) infections.6 In the era before direct-acting antivirals, it was used both as a monotherapy and in combination with interferon-alpha. Clinical trials showed that it could lead to a complete virological response in a subset of patients.8 However, with the advent of highly effective and well-tolerated direct-acting antiviral agents, the use of Tα1 for the treatment of viral hepatitis has become largely obsolete.8
In the context of human immunodeficiency virus (HIV) infection, Tα1 has been studied as an adjunctive therapy aimed at immune reconstitution. In combination with highly active antiretroviral therapy (HAART), Tα1 has been shown to be well-tolerated and to enhance the recovery of CD4+ T-cell counts, the primary target of HIV, thereby potentially accelerating the restoration of immune function in patients with advanced disease.14
3.2.3 The COVID-19 Experience
The COVID-19 pandemic prompted the investigation of numerous immunomodulatory agents, including Tα1, to manage the dysregulated immune response and severe lymphocytopenia characteristic of severe disease. The evidence base for Thymosin Alpha-1 research in COVID-19 is composed primarily of retrospective cohort studies, which have produced highly contradictory and conflicting results.
Several studies reported positive outcomes. For instance, one retrospective analysis of 76 severe COVID-19 patients found that Tα1 treatment was associated with a significant reduction in mortality (11.1% in the Tα1 group vs. 30.0% in the untreated group).29 The proposed mechanism was the restoration of T-cell numbers and the reversal of T-cell exhaustion, a state of T-cell dysfunction that occurs during chronic antigen exposure.29 Other reports suggested Tα1 could mitigate the “cytokine storm,” a hyper-inflammatory state implicated in severe COVID-19 pathology.18
In stark contrast, other retrospective studies found no benefit or even potential harm associated with Tα1 use. One study of 275 patients concluded that Tα1 had no beneficial effect on restoring CD4+ or CD8+ T-cell counts and was associated with a significantly longer duration of viral shedding.34 Another multicenter cohort study found that Tα1 use was associated with an increased non-recovery rate, particularly in patients with greater disease severity.35 This conflicting evidence underscores the significant limitations of retrospective, non-randomized data collected during a public health crisis and highlights the necessity of well-designed randomized controlled trials to determine the true efficacy of any intervention.
3.3 Vaccine Adjuvancy: A Consistent Signal of Efficacy
In contrast to the mixed results seen in other areas, the use of Tα1 as a vaccine adjuvant represents the most consistent and robust area of clinical evidence for its efficacy. An adjuvant is a substance added to a vaccine to enhance the magnitude and durability of the immune response. Tα1 is particularly effective in this role in populations known to have a suboptimal response to vaccination, such as the elderly, who experience age-related immune decline (immunosenescence), and patients with chronic conditions like end-stage renal disease requiring hemodialysis.9
Multiple clinical trials have quantified the significant benefit of Thymosin Alpha-1 research. For example, in a study of immunocompromised hemodialysis patients receiving an influenza vaccine, 71% of those who also received Tα1 achieved a seroprotective antibody titer (a four-fold or greater rise), compared to only 43% of those who received the vaccine with a placebo.16 The benefit was also demonstrated in hemodialysis patients who were non-responders to a previous course of the hepatitis B vaccine. Upon revaccination with the addition of Tα1, 64% of patients developed protective antibody levels, compared to only 17% in the placebo group.16
Beyond simply improving antibody levels, Tα1 has also been shown to translate this enhanced immunogenicity into improved clinical outcomes. In a large study of elderly individuals receiving the seasonal influenza vaccine, the group that received Tα1 as an adjuvant had a subsequent incidence of influenza infection of only 5.5%, a statistically significant reduction compared to the 19% infection rate observed in the group that received the vaccine and a placebo.16 This body of evidence provides strong support for the use of Tα1 as an effective strategy to improve vaccine efficacy in vulnerable populations.
Table 1: Summary of Key Clinical Trials of Thymosin Alpha-1
| Indication | Key Study Trial Name | Study Design | Research Population(N) | Treatment Regimen | Primary Outcome(s) | Key Results & Conclusion | Snippet Reference(s) |
| Metastatic Melanoma | Ruggiero et al. (2010) | Randomized, Multicenter, Phase 3 | 488 | DTIC + IFN-α +/- Tα1 at various doses | Best Overall Response @ 12 months | Did not meet primary endpoint. However, Tα1 arms showed higher response rates and a trend toward improved OS (9.4 vs 6.6 months). | 23 |
| Sepsis | TESTS Trial | Phase3, Multicenter, Double-Blind, RCT | 1089 | Tα1 1.6 mg SC BID vs. Placebo for 7 days | 28-day all-cause mortality | No significant difference in 28-day mortality. Subgroup analysis suggested potential benefit in patients ≥60 years and those with diabetes. | 25 |
| COVID-19 (Severe) | Liu et al. (2020) | Retrospective Cohort | 76 | Tα1 vs. No Tα1 | Mortality, T-cell counts | Tα1 treatment significantly reduced mortality (11.1% vs 30.0%). Associated with restored T-cell counts and reversed T-cell exhaustion. | 29 |
| COVID-19 | Wang et al. (2021) | Retrospective Cohort | 275 | Tα1 vs. No Tα1 | T-cell counts, Viral Clearance | Tα1 had no beneficial effect on restoring CD4+ or CD8+ T-cell counts. Tα1 use was associated with significantly longer duration of viral shedding. | 34 |
| Flu Vaccine Adjuvant (Elderly) | Gravenstein et al. | Double-Blind, RCT | 90 | Tα1 vs. Placebo biweekly for 4 weeks post-vaccination | Antibody Titer, Infection Rate | Tα1 group had greater immunization effect (69% vs 52%), and incidence of flu was significantly less in a separate study (5.5% Tα1 vs 19% Placebo). | 16 |
4. Potential Applications and Future Directions
4.1 Investigational Use in Autoimmune and Inflammatory Conditions
While Tα1 is primarily known as an immune-enhancing agent, a growing body of evidence suggests it may have a paradoxical therapeutic role in autoimmune and inflammatory diseases. This is based on the understanding that Tα1 functions as an immunomodulator, capable of restoring homeostasis rather than simply amplifying all immune responses. A key observation supporting this potential application is that patients with autoimmune conditions such as psoriatic arthritis (PsA), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) have been found to have significantly lower circulating levels of endogenous Tα1 compared to healthy individuals. This suggests that a relative deficiency of Tα1 may contribute to the immune dysregulation that underlies these diseases.
The mechanism for this potential benefit is thought to involve Tα1’s ability to promote the function of regulatory T-cells (Tregs). Tregs are a specialized subset of CD4+ T-cells that play a critical role in maintaining self-tolerance (preventing the immune system from attacking the body’s own tissues) and suppressing excessive inflammatory responses. By enhancing Treg function, Tα1 could help to dampen the aberrant autoimmune reactions that drive tissue damage in these conditions, thereby acting as a balancing agent to restore immune tolerance.
4.2 Theorized Benefits in Preventing Oxidative Damage
Beyond its specific effects on immune cells, Thymosin Alpha-1 research appears to possess broader cytoprotective (cell-protective) properties. Several studies have indicated that Thymosin Alpha-1 research can protect tissues from oxidative damage, a process implicated in aging and a wide range of chronic diseases.11 The mechanism involves reducing the cellular generation of reactive oxygen species (ROS), which are highly reactive molecules that can damage DNA, proteins, and lipids. Concurrently, Thymosin Alpha-1 research has been shown to increase the activity of the body’s own endogenous antioxidant enzymes, such as catalase and superoxide dismutase.14 This dual action—decreasing ROS production while bolstering antioxidant defenses—may contribute to its beneficial effects observed in various inflammatory and infectious conditions and represents an area for further investigation.
4.3 Novel Formulations and Targeted Delivery Systems
A significant limitation of many peptide therapeutics is their short half-life and lack of tissue specificity. Future research into Tα1 is likely to focus on peptide engineering and novel delivery systems to overcome these challenges. A compelling proof-of-concept is the development of Tα1-RGDR, a fusion peptide that covalently links Tα1 to an RGDR peptide sequence. The RGDR motif is known to bind to integrin and neuropilin receptors that are often highly expressed on the surface of tumor cells and tumor-associated blood vessels. Preclinical studies using this targeted construct have demonstrated that Tα1-RGDR achieves better accumulation in tumor tissues and exerts superior antitumor activity compared to unmodified Tα1. This strategy of creating targeted fusion peptides represents a promising avenue for increasing the therapeutic index of Tα1, maximizing its efficacy at the site of disease while minimizing potential systemic exposure and off-target effects.
5. Safety, Tolerability, and Regulatory Considerations
A striking feature of Tα1 is the apparent paradox between its extensive clinical use and favorable safety record in many parts of the world and its unapproved and restricted status in the United States. This discrepancy is not primarily due to direct evidence of toxicity but rather stems from differing regulatory standards, particularly concerning the robustness of efficacy data from large-scale trials and the quality control of bulk drug substances intended for compounding. Understanding this regulatory landscape is crucial for contextualizing the clinical potential of Tα1.
5.1 Comprehensive Safety Profile
The safety of Tα1 is remarkably well-established, supported by data from decades of clinical use. A comprehensive review of clinical trials involving over 11,000 human subjects, combined with post-marketing surveillance data from over 600,000 treated patients, consistently demonstrates an excellent safety and tolerability profile. Tα1 has been administered to a wide range of patient populations, including elderly individuals (up to 101 years of age), children, and various immunocompromised patients, with no significant harm or drug-drug interactions reported.
The most commonly reported adverse events are mild and transient, consisting primarily of local injection site reactions such as discomfort, redness, itching, or swelling that typically resolve within a short period. Unlike many other potent biological response modifiers, such as interferon or interleukin-2, Thymosin Alpha-1 research does not appear to induce the severe systemic toxicities, such as high fever, debilitating fatigue, or capillary leak syndrome, that often limit the use of those agents.
5.2 Pharmacokinetics of Subcutaneous Administration
Pharmacokinetic studies in humans have characterized the absorption, distribution, and elimination of Tα1 following subcutaneous administration. The peptide is absorbed rapidly, reaching maximum plasma concentrations (Tmax) approximately 2 hours after injection. It has a short serum half-life of about 2 hours, and studies have shown no evidence of drug accumulation even with daily dosing regimens. This predictable pharmacokinetic profile contributes to its ease of use and favorable safety.
5.3 The Global Regulatory Landscape and the U.S. FDA Stance
Thymalfasin, the synthetic form of Tα1, is an approved pharmaceutical product in over 35 countries, primarily in Asia, Latin America, and Eastern Europe. It is indicated for the treatment of conditions such as chronic hepatitis B and as an immune enhancer for various diseases.5
In contrast, Tα1 is not an approved drug in the United States and is not recognized in the U.S., European (except Italy), or Japanese Pharmacopoeias. The U.S. Food and Drug Administration (FDA) has taken a firm stance against its use, particularly in the context of compounded medications. The FDA’s decision not to include Tα1 on the 503A Bulks List (a list of bulk drug substances that can be used by compounding pharmacies) is based on several key concerns:
- Lack of Definitive Efficacy: The FDA’s review concluded there was insufficient high-quality evidence from large, well-controlled clinical trials to support the effectiveness of Tα1 for its proposed uses. The agency highlighted the inconclusive results from major trials in serious conditions like sepsis and melanoma as a primary reason for its position.37
- Poor Physicochemical Characterization: The agency has raised significant concerns about the quality of the bulk drug substance available for compounding. Specifically, it noted a lack of critical characterization data regarding impurities, aggregates, and solubility. This lack of a well-defined quality standard for the starting material poses a risk to patient safety.37
- Risk of Immunogenicity: As a peptide therapeutic administered via injection, Thymosin Alpha-1 research has an inherent risk of causing an unwanted immune response (immunogenicity). The FDA has stated that this risk could be amplified by the presence of uncharacterized peptide-related impurities and aggregates in poorly controlled compounded products.37
Reflecting these concerns, the FDA has actively issued warning letters and taken enforcement actions against compounding pharmacies and medical practices that have made unsubstantiated therapeutic claims about Tα1, particularly for the treatment of COVID-19, for which it is not approved.
6. Conclusion: Synthesizing the Evidence and Future Outlook
6.1 Recapitulation of Tα1’s Role as a Potent Immunomodulator
Thymosin Alpha-1 research has been unequivocally established as a potent, endogenous immunomodulatory peptide. Decades of research have elucidated its mechanism of action, revealing it to be a sophisticated regulator of immune homeostasis. It functions not as a blunt instrument of immune stimulation but as a precise orchestrator, initiating its effects by engaging the innate immune system through Toll-like receptors on dendritic cells. This primary interaction triggers a cascade of signaling events that leads to the maturation of these critical antigen-presenting cells, which in turn direct a robust and appropriately polarized Th1-type adaptive immune response. This ability to bridge the innate and adaptive immune systems and restore functional T-cell immunity is the cornerstone of its therapeutic potential.
6.2 A Nuanced Perspective on Clinical Efficacy and Unmet Potential
The clinical journey of Thymosin Alpha-1 research is a study in contrasts. Its safety profile is exceptionally benign and well-documented across vast patient populations. However, its clinical efficacy is highly context-dependent, a reality that has led to a complex and often contradictory body of evidence. The data supporting its use as a vaccine adjuvant are strong and consistent, demonstrating a clear clinical benefit in populations with compromised immune function. In contrast, its application in oncology and infectious diseases has yielded more ambiguous results. The definitive failure of the large-scale TESTS trial to show a mortality benefit in a broad population of sepsis patients stands as a cautionary tale against a “one-size-fits-all” approach to immunotherapy. Similarly, the conflicting retrospective data from the COVID-19 pandemic highlight the difficulty in assessing efficacy without the rigor of prospective, randomized trials.
6.3 Concluding Remarks on the Path Forward for Tα1 Research
The accumulated evidence suggests that the failure of Thymosin Alpha-1 research in large, heterogeneous trials should not be interpreted as a failure of the molecule itself, but rather as a failure of an undifferentiated clinical trial strategy. The path forward for Tα1 research must pivot from broad applications to a more precise, personalized medicine paradigm. The hypothesis-generating findings from subgroup analyses, such as the potential benefit in older or diabetic sepsis patients, strongly suggest that Tα1’s value lies in treating specific, well-defined states of immune dysfunction.
Future clinical trials should be designed to test this hypothesis directly. This will require the use of immune biomarkers—such as baseline lymphocyte counts, markers of T-cell exhaustion like PD-1, or baseline cytokine profiles—to stratify patients and enrich trial populations with individuals who are most likely to benefit from Tα1’s immune-restorative effects. By targeting patients with demonstrable immunosenescence, lymphocytopenia, or T-cell exhaustion, researchers can more accurately assess the therapeutic efficacy of Thymosin Alpha-1. This targeted approach holds the greatest promise for finally unlocking the full and considerable clinical potential of this foundational immunomodulatory peptide.
Works cited
- A Reappraisal of Thymosin Alpha1 in Cancer Therapy – Frontiers, accessed August 28, 2025, https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2019.00873/full
- A Reappraisal of Thymosin Alpha1 in Cancer Therapy – PMC – PubMed Central, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6742685/
- Modified Thymosin Alpha 1 Distributes and Inhibits the Growth of Lung Cancer in Vivo | ACS Omega, accessed August 28, 2025, https://pubs.acs.org/doi/10.1021/acsomega.0c00220
- Mechanism and clinical application of thymosin in the … – Frontiers, accessed August 28, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1237978/full
- Comprehensive Review of the Safety and Efficacy of Thymosin Alpha 1 in Human Clinical Trials | Timeless Health, accessed August 28, 2025, https://www.timelesshealthmd.com/scientific-publications/forbes-health-sewpr-wjhk2
- What is the role of Thymosin Alpha 1 (TA1)?, accessed August 28, 2025, https://www.droracle.ai/articles/134985/thymosin-alpha-1
- Thymalfasin: Uses, Interactions, Mechanism of Action | DrugBank Online, accessed August 28, 2025, https://go.drugbank.com/drugs/DB04900
- Thymosin alpha 1: A comprehensive review of the literature – Baishideng Publishing Group, accessed August 28, 2025, https://www.wjgnet.com/2220-3249/full/v9/i5/67.htm
- Thymosin alpha 1: A comprehensive review of the literature – ResearchGate, accessed August 28, 2025, https://www.researchgate.net/publication/346941286_Thymosin_alpha_1_A_comprehensive_review_of_the_literature
- e=”font-weight: 400;”>Thymosin Alpha-1 Peptide: Benefits and Safety – Innerbody Research, accessed August 28, 2025, e=”font-weight: 400;”>https://www.innerbody.com/thymosin-alpha-1-peptide</a>
- Thymosin alpha 1: A comprehensive review of the literature – PubMed, accessed August 28, 2025, https://pubmed.ncbi.nlm.nih.gov/33362999/
- Role of Thymosin Alpha 1 as an Immunoregulator Preventing Oxidative Stress and Cytokine Storm – DRSC | Healthcare Professionals, accessed August 28, 2025, https://www.drsrce.com/article-details-role-of-thymosin-alpha-1-as-an-immunoregulator-preventing-oxidative-stress-and-cytokine-storm
- Immune Modulation with Thymosin Alpha 1 Treatment – PubMed, accessed August 28, 2025, https://pubmed.ncbi.nlm.nih.gov/27450734/
- Safety and Efficacy of Thymosin alpha-1, An Immunomodulatory and Antiviral Therapy, accessed August 28, 2025, https://www.drsrce.com/article-details-safety-and-efficacy-of-thymosin-alpha-1,-an-immunomodulatory-and-antiviral-therapy
- Thymosin α 1 activates dendritic cells for antifungal Th1 resistance …, accessed August 28, 2025, https://ashpublications.org/blood/article/103/11/4232/17900/Thymosin-1-activates-dendritic-cells-for
- Influenza Vaccine Enhancement with Immunomodulating Peptide …, accessed August 28, 2025, https://www.biopharminternational.com/view/influenza-vaccine-enhancement-immunomodulating-peptide-thymosin-alpha-1
- Toll-Like Receptors – Abeomics, accessed August 28, 2025, https://www.abeomics.com/toll-like-receptors
- Thymosin Alpha 1 Mitigates Cytokine Storm in Blood Cells From Coronavirus Disease 2019 Patients – Oxford Academic, accessed August 28, 2025, https://academic.oup.com/ofid/article-pdf/8/1/ofaa588/36149798/ofaa588.pdf
- Thymosin-α1 modulates dendritic cell differentiation and functional …, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC1986709/
- pmc.ncbi.nlm.nih.gov, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7747025/#:~:text=The%20immune%20response%20of%20thymosin,it%20kills%20virally%20infected%20cells.
- The Effects of Thymosin Alpha-1 on Macrophages: A Cytological and Anti-Inflammatory Study – ResearchGate, accessed August 28, 2025, https://www.researchgate.net/publication/375248111_The_Effects_of_Thymosin_Alpha-1_on_Macrophages_A_Cytological_and_Anti-Inflammatory_Study
- Effect of thymosin alpha-1 on subpopulations of Th1, Th2, Th17, and regulatory T cells (Tregs) in vitro, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3854146/
- Large Randomized Study of Thymosin α 1, Interferon Alfa, or Both in …, accessed August 28, 2025, https://ascopubs.org/doi/abs/10.1200/JCO.2009.25.5208
- Evaluation of thymosin alpha 1 (Ta1) in nonclinical models of the immune-suppressing indications melanoma and sepsis – ResearchGate, accessed August 28, 2025, https://www.researchgate.net/publication/271772965_Evaluation_of_thymosin_alpha_1_Ta1_in_nonclinical_models_of_the_immune-suppressing_indications_melanoma_and_sepsis
- The efficacy and safety of thymosin α1 for sepsis (TESTS): multicentre, double blinded, randomised, placebo controlled, phase 3 trial | The BMJ, accessed August 28, 2025, https://www.bmj.com/content/388/bmj-2024-082583
- The efficacy and safety of thymosin α1 for sepsis (TESTS): multicentre, double blinded, randomised – The BMJ, accessed August 28, 2025, https://www.bmj.com/content/388/bmj-2024-082583.full.pdf
- Thymosin alpha 1: A comprehensive review of the literature – PMC, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7747025/
- Thymosin α 1 for treatment of hepatitis C virus: Promise and proof – ResearchGate, accessed August 28, 2025, https://www.researchgate.net/publication/44662012_Thymosin_a_1_for_treatment_of_hepatitis_C_virus_Promise_and_proof
- Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus …, accessed August 28, 2025, https://pubmed.ncbi.nlm.nih.gov/32442287/
- Thymalfasin (Thymosin Alpha 1) to Treat COVID-19 Infection (Ta1) – ClinicalTrials.gov, accessed August 28, 2025, https://clinicaltrials.gov/study/NCT04487444?intr=thymosin%20alpha%201&rank=9
- Thymalfasin (Thymosin Alpha 1) to Treat COVID-19 Infection (Ta1) – ClinicalTrials.gov, accessed August 28, 2025, https://clinicaltrials.gov/study/NCT04487444
- Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells – Oxford Academic, accessed August 28, 2025, https://academic.oup.com/cid/article-abstract/71/16/2150/5842185
- Thymosin Alpha 1 Mitigates Cytokine Storm in Blood Cells From Coronavirus Disease 2019 Patients – Oxford Academic, accessed August 28, 2025, https://academic.oup.com/ofid/article/8/1/ofaa588/6024458
- Thymosin Alpha-1 Has no Beneficial Effect on Restoring CD4+ and CD8+ T Lymphocyte Counts in COVID-19 Patients – Frontiers, accessed August 28, 2025, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.568789/full
- Efficacy of Thymosin Alpha 1 in the Treatment of COVID-19: A Multicenter Cohort Study – PMC – PubMed Central, accessed August 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8366398/
- Thymosin α1 continues to show promise as an enhancer for vaccine response | Request PDF – ResearchGate, accessed August 28, 2025, https://www.researchgate.net/publication/232227941_Thymosin_a1_continues_to_show_promise_as_an_enhancer_for_vaccine_response
- Thymosin alpha-1 (Ta1) related bulk drug substances – FDA, accessed August 28, 2025, https://www.fda.gov/media/183892/download
- FDA Provides Specific Compounded Drug Concerns Related to COVID-19 – GovDelivery, accessed August 28, 2025, https://content.govdelivery.com/accounts/USFDA/bulletins/2c3b1f7
- October 28, 2020 The Honorable Dr. Stephen M. Hahn Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver S – The Committee on Oversight and Accountability Democrats |, accessed August 28, 2025, https://oversightdemocrats.house.gov/sites/evo-subsites/democrats-oversight.house.gov/files/2020-10-28.RK%20to%20FDA%20and%20FTC%20re%20Thymosin%20Alpha-1.pdf
- FDA targets remdesivir, thymosin in compounding concerns – RAPS, accessed August 28, 2025, https://www.raps.org/news-and-articles/news-articles/2021/2/fda-targets-remdesivir-thymosin-in-compounding-con
- Paradigm RE LLC – 612014 – 12/07/2020 – FDA, accessed August 28, 2025, https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/paradigm-re-llc-612014-12072020
